Bacterial Conversations and Plaque Control: The Biofilm Pathogenesis Puzzle
In the dynamic world of microbial ecosystems, certain microbe communities engage in intricate interplay, influencing their surroundings with profound implications. The intricate processes support the bacterial interactions that forge resilient structures capable of challenging modern medicine’s defenses. Exploring these complex networks reveals their potential to illuminate innovative paths in managing these formidable biological entities.
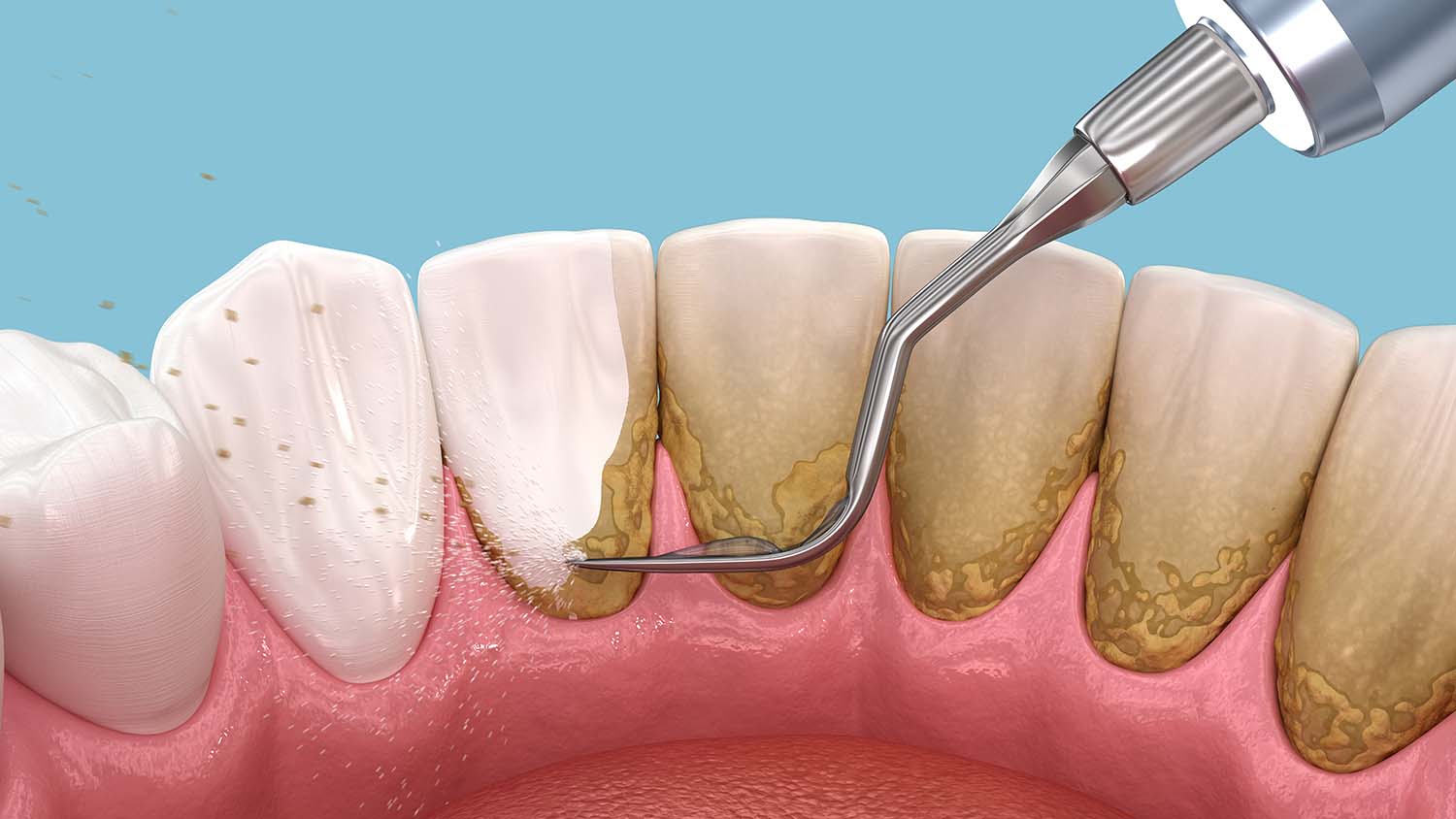
The Architecture of Microbial Fortresses
From Solitary Wanderers to Organized Communities
Contrary to the common perception of bacteria as solitary, drifting organisms, these microscopic entities possess a sophisticated ability to organize and collaborate. When specific pioneering colonizers, such as Streptococcus Mutans, adhere to a surface—be it a tooth, a medical implant, or living tissue—they initiate a transformation of their environment. They do not merely occupy space; they engineer it. These bacteria begin to secrete a slimy, glue-like substance known as an extracellular polymeric substance (EPS). This secretion acts as the mortar for their biological city, binding cells together and anchoring them firmly to the substrate.
This developing matrix is far more than a simple sticky trap; it is a functional, protective "fortress." Within this structure, the bacterial community is shielded from environmental fluctuations, dehydration, and mechanical shear forces. The formation of this barrier is the first critical step in pathogenesis. It allows the community to grow vertically and horizontally, creating a complex three-dimensional architecture that includes water channels to distribute nutrients and remove waste. This structural complexity turns a group of vulnerable individual cells into a unified, hardened entity that is exponentially more difficult to remove than its free-floating counterparts.
Chemical Conversations and Strategic Coordination
The construction of these microbial citadels is not a random occurrence but the result of precise communication. Bacteria utilize a sophisticated chemical language to coordinate their behavior, a process scientifically referred to as Quorum Sensing. Through this mechanism, microorganisms continuously release and detect signaling molecules. As the population density increases, the concentration of these signals rises. Once a specific threshold is reached, it triggers a synchronized genetic shift across the entire community. This "group vote" instructs the bacteria to ramp up the production of protective slime and virulence factors simultaneously.
This signaling network allows the community to act as a singular multi-cellular organism rather than a chaotic mob. It enables them to fortify their defenses before the host immune system detects a threat. For instance, if the colony senses external stress or antimicrobial agents, they can collectively alter their physiological state to bolster resistance. This level of coordination effectively outsmarts traditional medical interventions that assume bacteria act independently. By disrupting these communication lines, researchers hope to confuse the colony, preventing them from ever building their defensive walls in the first place, leaving them exposed and vulnerable.
The Mechanics of Persistence and Survival
Metabolic Adaptation and the Anaerobic Haven
Once the biofilm matures, it creates diverse micro-environments that cater to various bacterial needs. Deep within the thickened layers of the matrix, oxygen becomes scarce, creating a perfect sanctuary for Anaerobic Bacteria. Pathogens like Porphyromonas Gingivalis, which thrive in oxygen-deprived environments, flourish here. Protected by the upper layers of the biofilm, these anaerobes can proliferate undisturbed by the oxygen-rich environment of the human body that would normally inhibit them. This stratification allows strict anaerobes to survive in areas where they would typically perish, such as the supragingival regions of the mouth or shallow wounds.
Furthermore, life inside this dense matrix alters the metabolic state of the inhabitants. Bacteria deep within the structure often enter a state of dormancy or "sleep" due to nutrient limitation. Since most conventional antibiotics are designed to target and kill actively dividing cells—by disrupting cell wall synthesis or DNA replication—these dormant "persister" cells remain largely unaffected. They simply wait out the antibiotic storm. Once the treatment ceases and the concentration of the drug drops, these sleepers wake up, utilizing their preserved energy to repopulate the colony, leading to the recurring chronic infections that frustrate both patients and clinicians.
| Feature | Planktonic Bacteria (Free-Floating) | Biofilm-Associated Bacteria (Protected) |
| Susceptibility | High vulnerability to antibiotics and immune cells. | Up to 1,000 times more resistant to treatment. |
| Metabolic State | Active, rapid growth and division. | Variable; often dormant or metabolically slow. |
| Environment | Uniform exposure to oxygen and nutrients. | Complex gradients (pH, oxygen) creating diverse niches. |
| Defense Strategy | relies on rapid multiplication (flight). | Relies on physical shielding and community cooperation (fight). |
Synergistic Interactions and Immune Evasion
The resilience of these communities is further enhanced by the synergy between different species. In the context of oral health, for example, the relationship between early colonizers and late-stage pathogens is a prime example of biological cooperation. The matrix produced by sugar-metabolizing bacteria provides the necessary adhesion sites for more virulent strains to attach. This co-aggregation transforms a relatively benign accumulation into a pathogenic powerhouse. The combined output of these species includes enzymes that degrade host tissues and Bacterial Endotoxins that trigger severe inflammation.
This collaborative environment also actively subverts the host's immune response. The dense matrix physically blocks antibodies and immune cells like neutrophils from reaching the bacterial surface. Frustrated by their inability to engulf the invaders, immune cells may release cytotoxic compounds outside the biofilm. Paradoxically, this "frustrated phagocytosis" damages the surrounding healthy host tissue—causing the gum recession in periodontitis or the expansion of chronic wounds—while leaving the bacteria inside the fortress relatively unharmed. The bacteria essentially manipulate the host's own defense system to clear space for their further expansion, turning the body's protective measures into a tool for tissue destruction.
Overcoming the Barrier: New Frontiers in Management
The Limitations of Antibiotic Bombardment
Understanding the physical and biological properties of biofilms explains why the "kill everything" approach of standard antibiotic therapy often fails. The polymeric matrix acts as a molecular filter, physically retarding the diffusion of antimicrobial agents. Even if the drug penetrates the outer layer, the bacteria inside may employ efflux pumps—active transport proteins that pump toxic substances out of the cell—more efficiently when in a group. Consequently, the concentration of the antibiotic that actually reaches the target cells is often sublethal.
Exposure to sublethal doses of antibiotics is particularly dangerous as it encourages the development of resistance. The bacteria that survive are often the ones with genetic mutations that allow them to withstand the drug, and within the close quarters of the biofilm, these resistance genes can be easily swapped between neighbors through horizontal gene transfer. This renders the fortress not only stronger but also smarter. Therefore, treating biofilm-based infections requires a paradigm shift from simply poisoning the bacteria to dismantling their home. The focus is moving toward breaking the physical structure that provides them with such extraordinary advantages.
Breaking the Shield: Advanced Hygiene Strategies
To effectively combat these entrenched communities, the primary strategy must focus on physical disruption, a concept central to effective Plaque Control. Since chemical agents struggle to penetrate the matrix, mechanical removal—brushing, flossing, or professional debridement—remains the gold standard. By physically breaking the structure, the bacteria are forced back into their planktonic, vulnerable state, where they can be easily managed by the immune system or mild antimicrobials. However, mechanical removal is not always possible in deep tissues or on delicate medical implants.
This limitation has spurred the development of next-generation therapies designed to dissolve the matrix or inhibit its formation. Scientists are investigating enzymes that digest the specific proteins and sugars keeping the fortress walls intact. Other approaches involve using "quorum quenching" molecules that mimic bacterial signals to jam their communication lines, tricking them into remaining solitary and exposed. By combining mechanical disruption with these advanced chemical and biological tactics, we aim to strip the bacteria of their collective armor. Only by dismantling the fortress can we hope to win the battle against these microscopic collaborators.
| Approach | Mechanism of Action | Strengths & Limitations |
| Physical Disruption | Mechanically breaks the biofilm matrix (e.g., brushing, debridement). | Pros: Immediate removal; bacteria become vulnerable. Cons: Cannot reach microscopic irregularities or deep tissues. |
| Chemical Inhibition | Uses antiseptics or enzymes to dissolve the matrix or kill bacteria. | Pros: Can reach areas inaccessible to tools. Cons: Limited penetration; potential side effects on host cells. |
| Biological Interference | Disrupts signaling (Quorum Sensing) or uses phages. | Pros: Highly specific; low resistance risk. Cons: Still largely experimental; requires precise targeting. |
Q&A
-
What role does Streptococcus Mutans play in oral health?
Streptococcus Mutans is primarily associated with the development of dental caries (cavities). It metabolizes sugars from food to produce acid, which can demineralize tooth enamel, leading to decay. Effective oral hygiene and dietary control can help manage its impact on oral health.
-
How does Porphyromonas Gingivalis contribute to periodontal disease?
Porphyromonas Gingivalis is a key pathogen in the development of periodontal disease. It disrupts the immune response and contributes to inflammation and tissue destruction in the gums. Its presence in the oral cavity is a significant risk factor for periodontal disease, highlighting the importance of regular dental check-ups and plaque control.
-
What are anaerobic bacteria and their significance in oral infections?
Anaerobic bacteria are organisms that thrive in environments devoid of oxygen. In the oral cavity, they are often found in periodontal pockets and are significant contributors to infections such as periodontitis and dental abscesses. Managing these bacteria involves maintaining proper oral hygiene and possibly using antimicrobial treatments.
-
Can you explain quorum sensing and its relevance to bacterial behavior?
Quorum sensing is a communication mechanism used by bacteria to coordinate their behavior based on population density. It allows bacteria to regulate gene expression collectively, which can lead to increased virulence and biofilm formation. Understanding quorum sensing is crucial for developing strategies to disrupt harmful bacterial communities in the oral cavity.
-
What strategies are effective for plaque control to prevent oral diseases?
Effective plaque control involves a combination of mechanical and chemical methods. Regular brushing and flossing help remove plaque physically, while antimicrobial mouthwashes can reduce bacterial load. Dental professionals may also recommend scaling and root planing for patients with significant plaque accumulation to prevent oral diseases.
